This example will demonstrate how to determine the compressibility factor (z) for gas with CO2 and H2S. As it is described in the article,Determine Compressibility of Gases , it states that gas with CO2 and H2S must be corrected. This is similar to a normal method for determining z-factor but it is required some correction. Please follow the steps below;
Gas component is shown in Table 1

Table 1 – Gas Component
Reservoir pressure = 6,000 psia
Reservoir temperature = 190 F
1) Determine critical pressure and temperature of gas mixtures using Kay’s rule

Table 2 – Critical Pressure and Temperature
Pc‘ = Σyipci = 779.7 psia
Tc’ = ΣyiTci = -22.43 F = -22.43 +460 F = 437.57 R

Table 3 – Pc’ and Tr’ by Kay’s Rule
2) Determine ɛ

Where
A =sum of mole fractions of carbon dioxide (CO2) and hydrogen sulphide (H2S) in the mixture
B = mole fraction of hydrogen sulphide (H2S) in the mixture.
A =0.082 + 0.132 = 0.214
B= 0.132

3) Determine corrected pseudo-critical temperature (Tc”) and corrected pseudo-critical pressure (Pc”)
Corrected pseudo-critical temperature (Tc”)

Corrected pseudo-critical pressure (Pc”)

4) Calculate pseudo reduced temperature and pressure
Pr” = P ÷ Pc”
Pr” = 6,000 ÷ 729.94 = 8.22
Tr” = T ÷Tc”
Tr” = (190+460) ÷ (412.34)
Tr” = 1.58
Note: temperature must be in Rankin.
5) Read the compressibility factor (z) from the chart.
z = 1.022
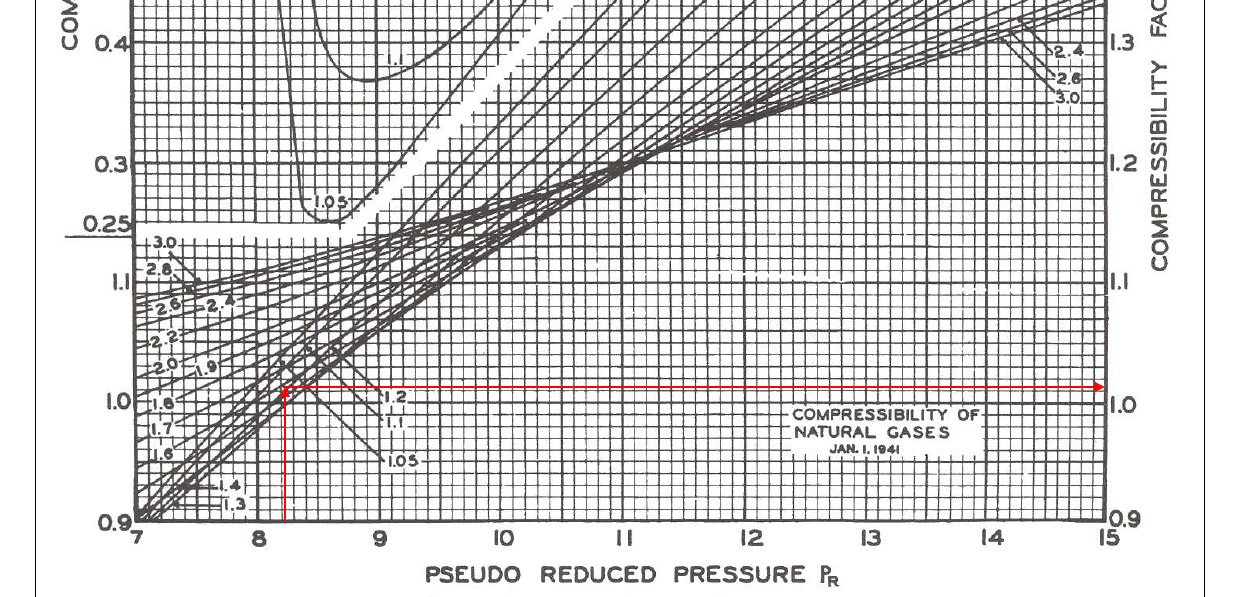
Figure 1-z-factor from the Standing and Katz Chart
References
Abhijit Y. Dandekar, 2013. Petroleum Reservoir Rock and Fluid Properties, Second Edition. 2 Edition. CRC Press.
L.P. Dake, 1983. Fundamentals of Reservoir Engineering, Volume 8 (Developments in Petroleum Science). New impression Edition. Elsevier Science.
Tarek Ahmed PhD PE, 2011. Advanced Reservoir Management and Engineering, Second Edition. 2 Edition. Gulf Professional Publishing.